A Kernel of Truth: Deconstructing the Miniature Pressure Vessel
From my analytical perspective, a popcorn kernel is a masterpiece of natural engineering. It’s not just a seed; it’s a tiny, organic pressure vessel, meticulously designed for a single, glorious moment of catastrophic failure. To understand why does popcorn pop, we must first dissect this little container. Inside its deceptively simple exterior lie three critical components:
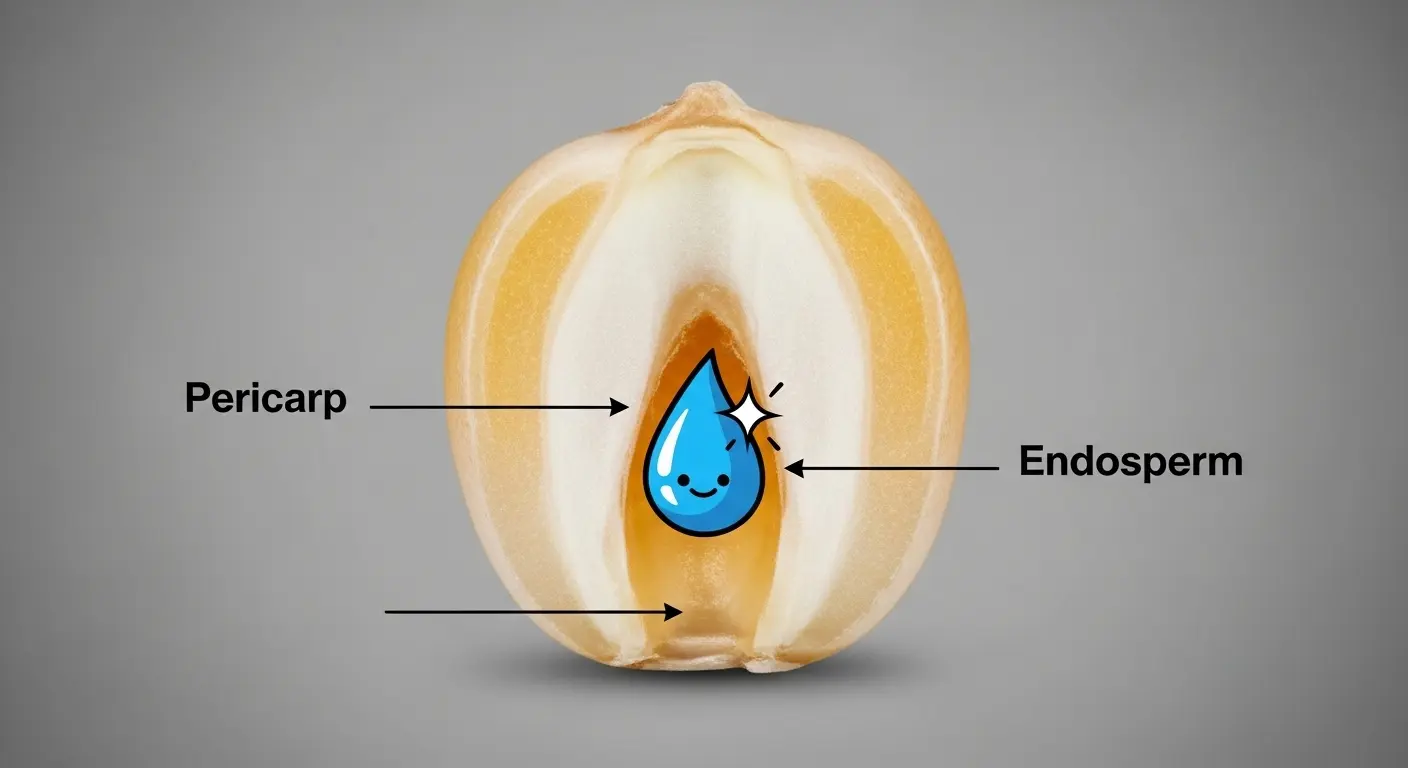
- The Pericarp: This is the hard, translucent outer hull. Think of it as the containment field. It’s remarkably strong and, crucially, non-porous. Its primary job is to hold everything together as the internal pressure skyrockets. No cracks, no glory.
- The Endosperm: This is the kernel’s payload, a starchy, protein-rich interior. It exists in two forms: a dense, glassy type and a softer, floury type. This starchy matrix holds the final, most important ingredient.
- The Water: Trapped within the endosperm is a minuscule amount of water, typically around 14% by weight. This isn’t just ambient moisture; it’s the catalyst, the explosive charge waiting for a spark.
These three parts work in perfect, violent harmony. The pericarp provides the confinement, the endosperm provides the fluffy payload, and the water provides the force. It’s a simple, yet brutal, system.
Heating Up: The Physics of Catastrophic (and Delicious) Structural Failure
When you apply heat to these tiny pressure vessels—be it in oil, hot air, or microwave radiation—the process begins. The heat transfers through the pericarp and begins to agitate that tiny droplet of water trapped within the starchy endosperm. As the temperature inside the kernel climbs past the boiling point of water (212°F or 100°C), something fascinating happens. Because the water is sealed within the high-integrity pericarp, it cannot simply boil off into steam. Instead, it becomes superheated liquid water.
This superheated water is under immense, growing pressure. As the temperature approaches approximately 355°F (180°C), the internal pressure can exceed 135 pounds per square inch (psi). That’s more pressure than you’d find in the tire of a large truck. The pericarp, for all its strength, has a breaking point. And when that point is reached, the failure is instantaneous and total.
This is the “pop.” It’s the sound of the hull breaching. In that split second, the superheated water flashes into steam, expanding to over 1,700 times its original volume. This explosive release of pressure turns the gelatinous, cooked starch of the endosperm inside out. The soft starch inflates rapidly and, upon hitting the cooler air, immediately solidifies into the wonderfully irregular, crunchy puff we call popcorn. It is a transformation born of violence.
The Unpopped and the Unworthy
Of course, not every soldier makes it through the battle. You always find a few unpopped kernels, which I refer to as “duds,” at the bottom of the bowl. Why do these fail to achieve their delicious destiny? My analysis points to two primary causes:
- A Compromised Hull: If the pericarp has even a microscopic crack or hole, the steam will leak out as it builds. Without the ability to contain and build up pressure, the kernel can never reach the critical point for a catastrophic explosion. It just sits there, getting hot and sad.
- Insufficient Moisture: The kernel might have a perfect hull, but if its internal water content is too low (either from improper storage or just being a genetic underperformer), there isn’t enough water to generate the steam pressure required for the pop.
So, the next time you’re enjoying a bowl of popcorn, take a moment to appreciate the elegant physics at play. Each piece is the beautiful, fluffy aftermath of a tiny, perfectly executed explosion. A testament to pressure, failure, and delicious transformation. A truly curious phenomenon.
